|
Eggbeater Jesus posted:Cheers for this, I had the old link and was going to share it with some friends but it was down for the count.
|
|
|
|

|
| # ? Jun 9, 2024 14:35 |
|
https://www.youtube.com/watch?v=qOSOXVSFdb0 This prep of sodium azide from hydrazine sulfate made me think of this thread. The videos in the channel are pretty good if you can stand the computer voice. From wikipedia: "Sodium azide has caused deaths for decades.[15] It is a severe poison. It may be fatal in contact with skin or if swallowed.[16] Even minute amounts can cause symptoms." "It produces extrapyramidal symptoms with necrosis of the cerebral cortex, cerebellum, and basal ganglia. Toxicity may also include hypotension,[19] blindness and hepatic necrosis"
|
|
|
|
DOOP posted:Sodium Borohydride is my favorite dangerous chemical, as my job is producing that suiff What do you feel about LiAlH4? Lithium Aluminium hydride! For when you really really really really really want electrons on everything. Rigged Death Trap has a new favorite as of 16:45 on Dec 23, 2015 |
|
|
|
LiAlH4 is an excellent propellant additive for parrafin wax based hybrid rocket fuel grains. It increases specific impulse, density, and fuel grain regression. And coating the LiAlH4 in parrafin wax is a great for keeping moisture or other problematic stuff away from your sweet fuel additive. Link.
|
|
|
|
funkatron3000 posted:https://www.youtube.com/watch?v=qOSOXVSFdb0 So when you're done synthesizing your super poison, what do you do with it. I can't imagine there's a particularly safe way to dispose of that.
|
|
|
|
KillHour posted:So when you're done synthesizing your super poison, what do you do with it. I can't imagine there's a particularly safe way to dispose of that. Sodium azide is used to inflate air bags in cars. It decomposes explosively to make nitrogen gas with a bit of heat. So disposing of it is not at all difficult.
|
|
|
|
Deteriorata posted:Sodium azide is used to inflate air bags in cars. It decomposes explosively to make nitrogen gas with a bit of heat. So disposing of it is not at all difficult. It decomposes to produce pure sodium, nitrogen gas, and a bit of heat. They have to put other things in the airbags to react with the sodium in order to remove it as a hazard.
|
|
|
|
Phanatic posted:It decomposes to produce pure sodium, nitrogen gas, and a bit of heat. They have to put other things in the airbags to react with the sodium in order to remove it as a hazard. Chlorine?
|
|
|
|
PerrineClostermann posted:Chlorine? Potassium nitrate and silica. You end up with fine bits of glass (without sharp edges).
|
|
|
|
You got your gasoline in my sulfuric acid! http://jalopnik.com/you-really-do-not-want-to-crash-into-a-truck-filled-wit-1749922658
|
|
|
|
quote:So when I was in grad school, one of the postdocs in my lab had to do a whole series of butane reactions. The trouble was that he needed an awful lot of butane, and the only good source was on another floor and too big to move.
|
|
|
|
Winchell Chung's "Atomic Rockets" site updated recently, with one of the additions being a section on a 1966 study by Rockwell on a capsule-style Mars lander that ran on a delicate balance of liquid almost-FOOF.Project Rho - Atomic Rockets posted:What is FLOX I hear you ask? Why, just a simple mixture of liquid oxygen, and Liquid Fluorine. There's a lot of rocketry math but it's an interesting read (especially for those who like rocketry math). Choice quotes include "the engine would periodically be sucking pure fluorine" and "a large melted crater with a few odd pieces of corroded metal and polished skeleton bits at the bottom".
|
|
|
|
quote:At least the MEM designers saved mass on the ignition system. You don't need any. FLOX/CH4 is hypergolic (because fluorine is hypergolic with almost anything). This is also a help when the ascent stage does staging, you can easily re-start the engines in mid-flight.
|
|
|
|
I'm assuming the reason they didn't go through with it because there's no way to make it not violently explode when the passivation layer suddenly breaks loose on take off.
|
|
|
|
Kwyndig posted:I'm assuming the reason they didn't go through with it because there's no way to make it not violently explode when the passivation layer suddenly breaks loose on take off.
|
|
|
|
Kwyndig posted:I'm assuming the reason they didn't go through with it because there's no way to make it not violently explode when the passivation layer suddenly breaks loose on take off. I would also imagine that having tons of liquid fluorine stored on-site would make it difficult to coax the ground crew to come to work in the morning.
|
|
|
|
I don't know about that, there's at least one company that still makes the stuff, so there's got to be some people out there willing to work with the devil.
|
|
|
|
Deteriorata posted:I would also imagine that having tons of liquid fluorine stored on-site would make it difficult to coax the ground crew to come to work in the morning. If work is still there.
|
|
|
|
Deteriorata posted:I would also imagine that having tons of liquid fluorine stored on-site would make it difficult to coax the ground crew to come to work in the morning. e. although lets be fair to the nickel, its usually the all the trimmings that come with a tank that fluorine will hate away first. zedprime has a new favorite as of 18:05 on Jan 12, 2016 |
|
|
|
Deteriorata posted:I would also imagine that having tons of liquid fluorine stored on-site would make it difficult to coax the ground crew to come to work in the morning. TBH that doesn't sound particularly less pleasant than other hypergolic fuels, and Russia and China use those pretty much exclusively. At least if the fluorine leaks it'll react with the environment pretty quick, unsymmetrical dimethylhydrazine and dinitrogen tetroxide being the big ones. Basically anything that makes a good rocket fuel is gonna be some scary poo poo.
|
|
|
|
zedprime posted:e. although lets be fair to the nickel, its usually the all the trimmings that come with a tank that fluorine will hate away first. Laughed a lot at this, explained to my wife what I was laughing at, explained this thread, linked her to the chemicals I won't work with blog. She's chuckling away in the corner now.
|
|
|
|
Abyssal Squid posted:TBH that doesn't sound particularly less pleasant than other hypergolic fuels, and Russia and China use those pretty much exclusively. At least if the fluorine leaks it'll react with the environment pretty quick, unsymmetrical dimethylhydrazine and dinitrogen tetroxide being the big ones. The dimethylhydrazine has a detailed description of its scent but the dinitrogen tetroxide article does not. Does it mean it is odorless? Inquiring minds need to know... Also, all this chat made me have another look at In the Pipeline and one of the recent articles there mentions mishaps with trimethylaluminium and trimethylindium which also sound like ever so pleasant compounds. http://blogs.sciencemag.org/pipeline/archives/2016/01/07/trimethylaluminum-explosion-in-massachusetts [edit] Also, from previous posts in this thread and the article I just linked it has become increasingly clear to me that working in many parts of the semiconductor industry is not an extremely attractive prospect.
|
|
|
|
Munin posted:The dimethylhydrazine has a detailed description of its scent but the dinitrogen tetroxide article does not. Does it mean it is odorless? Inquiring minds need to know... Dinitrogen tetroxide is colorless, but its odor is unknown because at atmospheric pressure it decomposes to nitrogen dioxide, which is dark red-brown with a sharp acrid odor, similar to chlorine or bromine.
|
|
|
|
Deteriorata posted:Dinitrogen tetroxide is colorless, but its odor is unknown because at atmospheric pressure it decomposes to nitrogen dioxide, which is dark red-brown with a sharp acrid odor, similar to chlorine or bromine. kk, I was curious since it mentioned astronauts being poisoned by some sort of leak. Presumably then it was a breakdown product that got them.
|
|
|
|
I never even took high-school chemistry (something I regret, but I did take physics at least) and I'm getting the hang of hearing names like 'trimethylaluminum' and turning that into knowing what's being talked about... this is kind of weird but also cool.
|
|
|
|
zedprime posted:You're skipping the step of making and storing tons of liquid fluorine in the first place, with amounts in one place which sound unprecedented today. Commercial synthesis is based on electrolysis, which means for commercial use its usually set up in a hand to mouth way because fluorine doesn't like staying in tanks, passivated nickel or not. I remember a lab in my college did experiments that involved fluorine gas. The tanks were kept in a isolated room and were filled with 90% helium to keep things from getting too out of hand.
|
|
|
|
Keiya posted:I never even took high-school chemistry (something I regret, but I did take physics at least) and I'm getting the hang of hearing names like 'trimethylaluminum' and turning that into knowing what's being talked about... this is kind of weird but also cool. Keep it up and you'll soon know which chemical names in movies are bullshit. Or not, because there's loads of weird trivial (non-systematic) names IRL that everyone uses.
|
|
|
|
Number-methyl-something usually means it wants to kill you too. Well there's a lot of stuff with methyls stuck to them that do indeed hate you.
|
|
|
|
Methyl groups on any sort of metal are like a VIP invitation to a party in your central nervous system where they get to trash the place.
|
|
|
|
Is that what someone meant on a forum once when he described methyl fluoronosulfate as "It also has this horrific tendency to methylate you. You do not want to be methylated." Or if not, what exactly happens to you when you get methylated?
|
|
|
|
Woolie Wool posted:Is that what someone meant on a forum once when he described methyl fluoronosulfate as "It also has this horrific tendency to methylate you. You do not want to be methylated." Well a bunch of stuff. You get to become partly biodiesel for one due to your lipids getting forcibly methylated. Also Methyl Fluorosulfonate is toxic in and of it's self. DemeaninDemon posted:Number-methyl-something usually means it wants to kill you too. Well there's a lot of stuff with methyls stuck to them that do indeed hate you. A lot yeah. But it ain't the methyl that screws things up. It's usually the thing it's attached to, the carbon just facilitates that by exploiting a Metallic element's weird electron situation. Ethyl/propyl/whatever metals are just as toxic. Metals do not like weakly electronegative bonds. Rigged Death Trap has a new favorite as of 16:35 on Jan 13, 2016 |
|
|
|
Rigged Death Trap posted:Well a bunch of stuff. Everyone wants to be coy. I want the horrible details!  http://blogs.sciencemag.org/pipeline/archives/2014/10/10/things_i_wont_work_with_peroxide_peroxides quote:ut there are wilder poly-peroxides out there. If you want to really oxidize the crap out of things with this compound, you will turn to the “peroxone process“. This is a combination of ozone and hydrogen peroxide, for those times when a single explosive oxidizing agent just won’t do. I’m already on record as not wanting to isolate any ozone products, so as you can imagine, I really don’t want to mess around with that and hydrogen peroxide at the same time. This brew generates substantial amounts of HOOOH, ozonide radicals, hydroxy radicals and all kinds of other hideous thingies, and the current thinking is that one of the intermediates is the HOOOOO- anion. Yep, five oxygens in a row – I did not type that with my elbows.
|
|
|
|
Deteriorata posted:Dinitrogen tetroxide is colorless, but its odor is unknown because at atmospheric pressure it decomposes to nitrogen dioxide, which is dark red-brown with a sharp acrid odor, similar to chlorine or bromine. I remember seeing a chart once (probably in this thread) of some compounds (alcohols maybe?) and their odors. A good third of the ones on the chart just had a skull and crossbones, aka "the concentration required to smell it would be high enough to kill you".
|
|
|
|
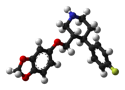
Woolie Wool posted:Well at least I know what it means to be oxidized. But what does it mean to be oximated?  Content: Does asbestos count? Not that it hasn't been brought up before, but it is indeed my F dangerous, uh, substance. The concept of something fine enough to cut DNA is terrifyingly fascinating and unlike a lot of contributions to this thread it's still around the place in situations any unlucky Average Joe could encounter it.
|
|
|
|
Asbestos did nothing wrong. Although its probably safe to file away with stuff like DDT and CFCs as materials too beautiful for the human decision making process.
|
|
|
|
Luneshot posted:I remember seeing a chart once (probably in this thread) of some compounds (alcohols maybe?) and their odors. A good third of the ones on the chart just had a skull and crossbones, aka "the concentration required to smell it would be high enough to kill you". There's this, which also labels some chemicals as "unique" in odor. Woolie Wool posted:Everyone wants to be coy. I want the horrible details! ETA: Actually, last time this came up, it was also you asking for horrible details. So come on, chemists, do tell us more. darthbob88 has a new favorite as of 18:22 on Jan 13, 2016 |
|
|
|
https://fat.gfycat.com/EarnestEqualAlpinegoat.webm source: https://www.youtube.com/watch?v=l285JDSiOOo `Nemesis has a new favorite as of 19:05 on Jan 13, 2016 |
|
|
|
darthbob88 posted:There's this, which also labels some chemicals as "unique" in odor. A methyl group is a carbon with three hydrogens on it, with the fourth available bond on the carbon sticking to whatever's been methylated. Methyl groups are sizable compared to their mass and nonpolar. If cell walls get methylated, the lipids don't fit together very well and the membrane starts leaking; if DNA gets methylated when it shouldn't be (DNA methylation is a common reaction in many forms of life, but only at specific spots on the genome), the various proteins that read it and do things to it bump into the methyl group and can't go any further; anything that relies on being polar to participate in enzymatic reactions will stop working if the ligand or enzyme gets methylated. It's kind of hard to nail down exactly what goes wrong with methylation because A. it's like dumping ball bearings covered in glue into a car engine, something's gonna get weird but who the hell knows what, and B. usually the methyl group itself is not nearly as dangerous as what it's attached to, because methyl groups being nonpolar/not very dense/relatively small mean they happily float through lipid membranes and anything else nonpolar. As people have mentioned, this is basically a VIP ticket for whatever's attached to go hang out and party inside the nearest available cell. For example, you might have seen pictures of people sitting on liquid mercury and being fine, but dimethylmercury is one of the most powerful neurotoxins known to science. The famous case of Karen Wetterhahn is the classic example - she spilled a couple drops of dimethylmercury onto her latex-gloved hand, cleaned it off after maybe a minute, and spent the remaining year of her life in a vegetative state. The methyl groups helped the mercury diffuse through the glove, through her skin, and into her body in the space of twenty seconds tops, and from that point on she was doomed.
|
|
|
|
Rigged Death Trap posted:Also Methyl Fluorosulfonate is toxic in and of it's self. 
|
|
|
|

|
| # ? Jun 9, 2024 14:35 |
|
I have discovered that there is a brand of woo involving breathing singlet oxygen to supposedly increase fitness performance 
|
|
|