|
Pellisworth posted:
Ahh yep, my bad, that's what I was thinking when I was referring to pH optimization was bringing things into the range where you can use calcium or other ions to bring down solubility. Removing bicarbonate would be as simple as having an immobilized enzyme with a mobile reaction phase bringing in water that is not saturated with bicarbonate, while drying the saturated water product in a separate chamber (you could use seawater as you suggested, since it seems to be a great source of counterions). Recapture the water and you can have a self-contained system that only requires the addition of counterions. I'm not sure where you're thinking we're losing efficiency at if we're only going to bicarbonate (other than the issue of heat for evaporation...) - CO2(g) -> HCO3(aq) is just enzymatically accelerated, and pairing with a counterion isn't an energetically demanding process. The biggest issue with bicarbonate is having to dry it to take it out of the system, but if you use something like a nuclear powerplant (where excess heat is always an issue), it seems a bit more feasible. As well, most other plant designs (coal and natural gas) use turbines, which have plenty of waste heat that could be used. CO2 fixation using enzymes is a big DOE project right now, and grant proposals have been written that would use carbonic anhydrase (example: http://www.netl.doe.gov/publications/proceedings/01/carbon_seq/5a5.pdf - first one that came up in google). The lab I work in currently (not the one in the pdf) is a potential recipient of the money, so it's a project close to my own heart. I know that resources are limited in ocean water, but zinc is pretty easy to come by terestrially (and we're definitely interested in exotic metals to do this, as they may be faster than Zn). What we'd ideally like to do generate bicarbonate with one enzyme, and then deprotonate bicarb in another and bind it to calcium or another ion to make a poorly soluble product like lime (or ideally do CO2 -> HCO3 -> CO3 -> CaCO3 all in one protein, which might be possible). Or, of course, we could take HCO3 generated in the CA and perform some other C-C bond-forming reaction, which would be ideal (but a bit harder). There are candidates for this, since bicarbonate is the product of lactate decomposition for example, and anabolic pathways in other organisms can take us in the opposite direction and incorporate HCO3 into energetic molecules. BobTheFerret fucked around with this message at 05:37 on Dec 7, 2011 |
|
|
|

|
| # ? Apr 30, 2024 19:27 |
|
BobTheFerret posted:Just for anyone who's not up on what modern chemistry/biochemistry is cooking up to solve the problem of excess CO2, it might not be completely unreasonable to say we could have a way to fix massive amounts of CO2 in the next 5-10 years, assuming the powers that be are willing to throw money at the development of what has already been discovered. How cost effective and scalable are these methods? My understanding is that the proposed solutions either didn't pan out (iron fertilization), are prohibitively expensive (any space solution), can't be done fast enough (going nuclear), don't scale (direct air capture) or can't be done fast enough (tera preta production) Do these have those problems negated? It would seem to me as the rate of carbon introduction to rate of capture would be a problem, as would limited supplies of copper
|
|
|
|
Pellisworth posted:
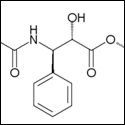
The mechanism is pretty cool - it's literally direct bond formation between two bound CO2 molecules by formation of a CO2- radical. Uses only 4 electrons. HCl is not hard to get, but is actually not needed (they also use LiClO4, Li+ as a counterion), and in their system, the reduction potential for the bond-forming step is -0.03V vs. NHE (hydrogen electrode), which is damned good. Their mechanism using Li+ counterion actually allows them to recover all counterion, meaning that they only end up using electrons in the reaction. Here's a picture of the reaction mechanism for any interested parties. It's amazingly simple:  I'm not handwaving away the energy requirement at all in my original post - that's what I see as being the main issue. We could take out tons of CO2 as it stands right now, but there has to be a willingness to spend the money on generating energy to do it (in terms of dedicated or paired fixation systems).
|
|
|
|
BobTheFerret posted:Ahh yep, my bad, that's what I was thinking when I was referring to pH optimization was bringing things into the range where you can use calcium or other ions to bring down solubility. Removing bicarbonate would be as simple as having an immobilized enzyme with a mobile reaction phase bringing in water that is not saturated with bicarbonate, while drying the saturated water product in a separate chamber (you could use seawater as you suggested, since it seems to be a great source of counterions). Recapture the water and you can have a self-contained system that only requires the addition of counterions. I'm not sure where you're thinking we're losing efficiency at if we're only going to bicarbonate (other than the issue of heat for evaporation...) - CO2(g) -> HCO3(aq) is just enzymatically accelerated, and pairing with a counterion isn't an energetically demanding process. The biggest issue with bicarbonate is having to dry it to take it out of the system, but if you use something like a nuclear powerplant (where excess heat is always an issue), it seems a bit more feasible. As well, most other plant designs (coal and natural gas) use turbines, which have plenty of waste heat that could be used. Ah ok, yeah that makes a lot more sense, thanks! Now the science nerd gears in my head are spinning, something like that might work pretty well. Especially if you found a CA from a thermophilic organism, CaCO3 is more insoluble at higher temps. Still doesn't solve your problem of getting from bicarb to carbonate, though. You might look into the biochemistry of shell-forming marine critters. You wouldn't necessarily have to maintain reaction conditions at low enough pH that all your bicarb converts to carbonate. In fact that might be a bad idea, low pH is no good for calcite formation. You just want your rate of calcite precipitation to equal your rate of CO2 conversion to bicarb. How you do that, don't ask me  I don't usually think much about practical applications of these things :P I don't usually think much about practical applications of these things :PEdit: yeah, that oxalate reaction is pretty slick. It's just a matter of how you generate those 4e- per CO2 molecule sequestered. Have you done any reading on the more primitive CO2 fixation pathways? I'm not super familiar with them, but there are several fairly unusual and recently discovered biochemical alternatives to the Calvin cycle. I have no idea if there's much inspiration there for engineering CO2 sequestration reactions based on those, but nature has a way of coming up with some pretty awesome stuff all on its own. http://en.wikipedia.org/wiki/Carbon_fixation#Other_autotrophic_pathways Pellisworth fucked around with this message at 06:03 on Dec 7, 2011 |
|
|
|
Fried Chicken posted:How cost effective and scalable are these methods? My understanding is that the proposed solutions either didn't pan out (iron fertilization) BobTheFerret fucked around with this message at 06:11 on Dec 7, 2011 |
|
|
|
Pellisworth posted:Especially if you found a CA from a thermophilic organism. Do you mean high pH (low proton concentration maybe is what you were thinking)? I wasn't sure about that in your last post. pH stability is important, but actually, the internal pH in a protein can be way different from external, so as long as we perform all steps within the protein (from CO2 to CaCO3). Hypothetically we can just use amino acid residues in the active site to deprotonate HCO3 and have a calcium ion bound nearby as well as saturating Ca2+ in solution.
|
|
|
|
Killin_Like_Bronson posted:How do you get the guy on his last week of work do his share? He's leaving and he knows it, the company be damned. Religion can work against it as Jesus will be here any day now. So in conclusion, I don't know. It definitely is hard to accomplish something when a large group of the electorate are of the opinion that they are in the process of bringing about a prophecy they think is necessary for them to get to heaven. It really brings the question to mind of, "How hosed up do you have to be, to think that bringing about the death of billions of human beings is a WIN for your religion that will give you eternal life?" Actions that completely go against the preachings of your own main prophet.
|
|
|
|
Fried Chicken posted:My understanding is that the proposed solutions either didn't pan out (iron fertilization)copper Short answer: yes, iron fertilization of the oceans is a terrible idea. I could write a gigantic post on this if there's interest but no one in the thread seems to be suggesting it as a serious option so I won't invest the effort unless it's desired. BobTheFerret posted:Do you mean high pH (low proton concentration maybe is what you were thinking)? I wasn't sure about that in your last post. pH stability is important, but actually, the internal pH in a protein can be way different from external, so as long as we perform all steps within the protein (from CO2 to CaCO3). Hypothetically we can just use amino acid residues in the active site to deprotonate HCO3 and have a calcium ion bound nearby as well as saturating Ca2+ in solution.
|
|
|
|
Yiggy posted:How the hell do we get past that sort of apathy? And rationally, if you're one of those people... why should you suffer if we're going to hell in a handbasket, and you won't experience any of the heat? Resources exist to be consumed. And consumed they will be, if not by this generation then by some future. By what right does this forgotten future seek to deny us our birthright? None I say! Let us take what is ours, chew and eat our fill. E: quote:Short answer: yes, iron fertilization of the oceans is a terrible idea. I could write a gigantic post on this if there's interest but no one in the thread seems to be suggesting it as a serious option so I won't invest the effort unless it's desired. I'd appreciate it if you expanded on this, as I'm curious. I've heard it bandied around before as a possible solution, but I'm not too familiar with the particulars.
|
|
|
|
RPZip posted:I'd appreciate it if you expanded on this, as I'm curious. I've heard it bandied around before as a possible solution, but I'm not too familiar with the particulars. Ok, this will be a slightly abbreviated version so as not to turn into a rant-length diatribe (what I do for a living is intimately tied to iron fertilization and I've spent a great deal of time studying it): Starting in the 90s, oceanographers realized that large parts of the world's oceans are deficient in iron (Fe hence). If you added some Fe, they reasoned, you should be able to stimulate blooms of phytoplankton (algae) who would then die, sink to the bottom of the ocean, and thus sequester all that juicy CO2 (now in the form of organic matter) at the bottom of the ocean. Several field experiments were conducted to test this hypothesis, several of which seemed to sort of work and the rest of which failed miserably. The consensus that has developed over the last decade (and is pretty much universal in the last ~5 years) is that Fe fertilization is just plain not a workable idea, though we learned a lot about the ocean in the process of studying it. Problems: 1. Logistics. Most of the regions of the ocean that are Fe-deficient are in the middle of nowhere. It's not as simple as dumping Fe into the coasts, as virtually all coastal oceans have plenty of Fe and other nutrients are the limiting factor (such as nitrogen or phosphorus). You'd need to cart many boatloads of Fe to (for example) the Southern Ocean near Antarctica, or the Bering Sea off of Alaska. I've seen several projections using very optimistic sequestration yields and all of them concluded it's basically a net loss or very minor gain of CO2 sequestered versus CO2 generated in shipping and dumping the iron out that far to sea. 2. Efficiency. Somewhat ironically, the guy that first realized the importance of Fe in the oceans (John Martin) is also very well known for his studies of CO2 export to the deep oceans. It's called the "Martin curve" and it's an exponential curve describing the percentage of organic CO2 that's fixed by organisms near the surface that makes it to a certain depth. About 1% of carbon is exported from the surface ocean to the deep, and about 0.1% overall ends up buried in ocean sediments. 0.1% is, of course, a laughably lovely sequestration efficiency. 3. Sustainability. You can't just dump Fe in forever and expect the same yields, obviously. At some point you'll deplete the other nutrients required for life. Organisms need things like nitrogen, phosphorus, silicon (in the case of diatoms whose shells are made of it), and other metals to live, and those elements are also in short supply. In fact, most of the ocean is nitrogen-limited, Fe-limited regions are the exception rather than the rule. You might get some response initially, but eventually you'd have to start fertilizing with nitrogen to maintain your algae yields. And phosphorus. Etc. 4. Organism specificity. This kind of relates to efficiency, but only certain kinds of algae are good at sequestering carbon. You want to trigger gigantic blooms of critters that form hard calcium carbonate shells. Those shells then sink to the bottom largely intact and get buried in the ocean floor (and eventually form limestone, huzzah!). Here's the problem. We have exactly zero control over what organisms Fe fertilization causes to bloom. In fact, in the field experiments where Fe fertilization successfully triggered blooms, by far the most abundant organism that grew was Pseudonitzschia, a diatom (which has silica, not carbonate shells--no good for CO2 sequestration!) which normally lives encrusted on the gills of fish and also is known to produce domoic acid, which causes amnesic shellfish poisoning in humans. OOPS
|
|
|
|
The biggest problem with carbon sequestration is that there's no money in it, and noone will touch it until then. By the time any sort of energy market will respond to that, it will be far too late. My employer's thrown billions of dollars at alternative energy research and our CEO has directly said that the economics of climate change basically ensures that the externalities of anthropogenic climate change will gently caress the planet up beyond belief before there is any kind of market-based solution. If we spent the money we dumped on the post-9/11 war on terror or whatever on some sort of Manhattan project to reduce carbon emissions through a huge portfolio of wind/biomass/geothermal/whatever we might have been able to stay under that 2C limit, but we didn't. Edit: That being said, those enzymatic pathways for CCS sound loving awesome, even if you do have to make them work at 80-100C, I do that all the time at work Bastard Tetris fucked around with this message at 07:36 on Dec 7, 2011 |
|
|
|
Pellisworth posted:Ah ok, yeah that makes a lot more sense, thanks! Now the science nerd gears in my head are spinning, something like that might work pretty well. Especially if you found a CA from a thermophilic organism, CaCO3 is more insoluble at higher temps. Still doesn't solve your problem of getting from bicarb to carbonate, though. You might look into the biochemistry of shell-forming marine critters. You wouldn't necessarily have to maintain reaction conditions at low enough pH that all your bicarb converts to carbonate. In fact that might be a bad idea, low pH is no good for calcite formation. You just want your rate of calcite precipitation to equal your rate of CO2 conversion to bicarb. How you do that, don't ask me Jesus. Now I actually know how it feels to be a moron, but I feel slightly better listening to the two of you hash this out.
|
|
|
|
So what can someone breaking into environmental science expect their future to be? Trying to keep food and water available? Cleaning things up? Impotently warning ignorant masses? Only keeping the rich in a clean place while living on their land and hiding your face from the starving, sickened masses? Really. I like water and I'd like to think I'd be keeping people from dying of thirst more than just keeping irrigation available for the rich at the expense of the poor.
|
|
|
|
Yiggy posted:So, I'm 27 years old. I will be dead before most of the Really Bad Stuff hits the fan. Now, I believe global warming is real, and its happening, and things are going to get terrible. But, this is the problem I run into whenever I try to talk to anyone about this in an older generation. They never outright, explicitly say it, but its ultimately the major hang up in the argument. "Ok, so what if you're right? That won't effect me." Its the largest barrier to getting them on our side. If they're religious, maybe you can make an appeal al la E.O. Wilson's The Creation. Or maybe if they have grand children, maaaaybe you can pluck some heart strings and get them to think about the next generation. More often then not, older people I talk to just can't be brought to care. Because its really never going to affect them. I'm 27 too and I can expect to live until I'm about 80, so that takes us both up to the 2060s or even the 2070s. As I understand it the seriously hosed up poo poo will start happening at about 2050, so we will both live long enough to know whether human civlisation will survive the century. Our retirement's are going to be pretty poo poo though. On the positive side you don't need to worry too much about your 401k or super plan or whatever because it'll probably have evaporated or been plundered by the financial classes long before then.
|
|
|
|
NoNotTheMindProbe posted:I'm 27 too and I can expect to live until I'm about 80, so that takes us both up to the 2060s or even the 2070s. As I understand it the seriously hosed up poo poo will start happening at about 2050, so we will both live long enough to know whether human civlisation will survive the century. Thats all well and good, but convincing the 27 year olds isn't really the issue. (Also I pretty much agree with you for what its worth). Yiggy fucked around with this message at 15:11 on Dec 7, 2011 |
|
|
|
Out of morbid curiosity, I jumped on RealClimate to see what's what. Apparently, a new paper was published not long ago that shows new data on El Nino and volcanic eruptions. These variables were causing a net decrease in stats regarding surface temperature (I think), leading to the deniers claiming that global warming stopped in 1998. The adjusted variables show that global warming is not only continuing, it's accelerating, as 2010 was the strongest La Nina year on record. Edit: actually, what really caught my eye was the comment section on New Scientist, where an army of trolls calling themselves "wombats" attacked the results with predictably bad smears. Check it out. Kafka Esq. fucked around with this message at 17:15 on Dec 7, 2011 |
|
|
|
PainterofCrap posted:Jesus. Now I actually know how it feels to be a moron, but I feel slightly better listening to the two of you hash this out. Damnit, it isn't that hard! You can do it too, it just takes some time and a little bit of work. This is no way my specialty, I just spent about an hour or so learning (and occasionally refreshing) the basics of it and you can too. Don't sell yourself short, other people will do that for your for free.
|
|
|
|
My little sister was a climate researcher at DOLA , and a stint at the CSIRO,in australia, specializing in ground water modelling re climate change. She left because she felt there was too much pressure on her to report positive happy outcomes when all the data and modelling kept basically shouting DOOM DOOM DOOM out of the maths. Her take was that it only requires a very small, tens of centimeters, broad change in ocean levels to utterly saline the gently caress out of ground water supplies due to, uh, some sort of funky salt equilibrium fluid mechanical dinglewidgety thing I dont understand. I think the salt water in the ground water wants to have some sort of equilibrium with the ocean or something, and if it rises it seeps into the fresh water pockets and uh something bad. But everytime her team went to publish she'd get blowback from non-scientific admistrator types who'd want to drown the research in committees and poo poo. This , by the way was during the conservative howard regime, so it might have improved somewhat during labor. It got too much for her, she felt we have a tiny window of opportunity to do something about it before australia basically loving loses its water supply and renders the continent extremely hostile to food security, and she noted that already much australian farmland had been lost to salinity and there was a growing feeling the old explainations of "Well, it was bad farming practice" didn't match the data well at all, whilst climate change during the periods from early industrial expansion onwards seemed to better match it , somehow which again I dont really understand. Needless to say, she quit, and took a cushy job in the UK as a consultant to companies looking to go "green", since she felt the political climate is better there and in her words "The UK is already hosed, we can only make it better, Australia isn't completely hosed yet, and its depressing as gently caress watching it get hosed". Oh add to that crank death threats, columnists writing news stories about her collegues just making fabricated poo poo up about them, fuckwit right wingers protesting at her office. She's a loving scientist, a dorky pocket-calculator girl, not a frigging rough-as-nuts politician. She never signed on to be literal hitler IRL to half the loon population. Plus she privately thinks we're hosed no matter what we do, all thats a variable now is divining the specific nature of the mess ahead. I'll add she goes loving feral when people claim climate scientists report bad news because its politically expedient. The actual experience of climate scientists is actually a total nightmare of uncovering a slowly evolving disaster amidst a slickly funded political climate that wants their very research field dead, dead and dead. gently caress denialists. Failed human oxygen theives each and every one of them.
|
|
|
|
As the impacts of climate change hit, particularly talking about food supply but weather too, hundreds of millions of people will die. This means hundreds of millions of people will stop consuming fossil fuels etc, reducing carbon emissions. How many people have to die before the climate stabilizes? Assume the poor ones who use little energy go first. This is exactly what is going to happen, so I hope someone has done some research.
|
|
|
|
sanchez posted:As the impacts of climate change hit, particularly talking about food supply but weather too, hundreds of millions of people will die. This means hundreds of millions of people will stop consuming fossil fuels etc, reducing carbon emissions. How many people have to die before the climate stabilizes? Assume the poor ones who use little energy go first. Since we need an 80% worldwide emission reduction... all of them? We'll probably get our collective heads out of our asses after the first billion, so maybe one or two more after that until we fully adapt?
|
|
|
|
http://www.guardian.co.uk/environment/2011/dec/07/public-support-climate-change-declinesquote:here has been dramatic decline over the past decade in the public's support for tackling climate change in Britain. Backing for higher green taxes and charges has waned and scepticism about the seriousness of the threat to the environment has increased. No doubt some of this is due to the denialists campaign, but I also think some of the proponents of climate change share this responsibility. Every time thereís a heat-wave we seem to see a lot of people blame it on global warming, and here in Australia the head climate scientist, Dr Tim Flannery, warned of our cities running out of water right before we were deluged with some of the heaviest rains in decades. Iím also not surprised that opposition to action is stronger amongst the working class, who already suffer from job security issues in heavily carbon-emitting industries and are probably correct that it would be them who suffer from a reduction in carbon emissions rather than the wealthy. Pipe Dreamer fucked around with this message at 00:09 on Dec 8, 2011 |
|
|
|
You know, all the science talk in this thread is bringing back that Earth 2100 special from a couple (few at this point?) years back. That was drat scary and so many people just poo poo'd it away.
|
|
|
|
Heh. Maybe the rich and elite know we're hosed and this last decade has been a free for all to horde as much as possible to prepare for the fuckedness.
|
|
|
|
Gatts posted:Heh. Maybe the rich and elite know we're hosed and this last decade has been a free for all to horde as much as possible to prepare for the fuckedness. That's the thing though, they really haven't. This isn't going to be about money, the dollars they have will collapse with the rest of it. Its about potential and kinetic energy in its purest form, the ability to move things from place to place and grow stuff and then ship it to wherever they need it. All of that goes away if our system breaks down. People really don't understand how delicate stuff like logistics, modern agriculture, aquaculture and manufacturing are. The things that are taken for granted to make all of the former things work..go away..in the case of a massive breakdown and we regress back to pre-industrial times. Yeah sure, you can pay men with guns to protect you..but what are you going to pay with? Food? How long is that going to work before they just look at you as an energy liability? And I'm not talking about black gold here, I'm talking about energy one needs to sustain their own lives. No, there will be no technologic cocooned elite living in arcologies while the other 7.9 billion of us starve...no no, its much more sinister than that.
|
|
|
|
sanchez posted:As the impacts of climate change hit, particularly talking about food supply but weather too, hundreds of millions of people will die. This means hundreds of millions of people will stop consuming fossil fuels etc, reducing carbon emissions. How many people have to die before the climate stabilizes? Assume the poor ones who use little energy go first. There are positive feedback effects that keep this from happening. Once we cross a certain threshold, we are hosed, even if we reduce carbon emissions to zero afterwards. The Earth will probably recover on a time scale of thousands of years, but there is no recovery on a shorter time scale.
|
|
|
|
This thread is making me depressed, but it really sounds unbelievable that people are suggesting catastrophic scenarios in the next 20 years and literally no one is thinking about it, which does give me some doubt to these claims. I'm not saying Climate Change doesn't exist, I'm just asking if its gonna really get apocalyptic-level
|
|
|
|
|
Does anyone have some information on the accuracy of the various IPCC assessment report predictions? I've been discussing global warming with two co-workers for a while now. They're at fairly different ends of the spectrum regarding global warming - one is taking a really stubborn stance ("we don't know what's happening and there's no way to know what's happening. Also Climategate.") while the other accepts the scientific literature behind it but is skeptical of the ability of models to predict the impact of greenhouse gasses. When I was talking to the latter, we realized neither of us actually had any data to back up our assertions - models can be accurate vs. models are rarely accurate - so we just called it a truce until we could generate some evidence.
|
|
|
|
SGRaaize posted:This thread is making me depressed, but it really sounds unbelievable that people are suggesting catastrophic scenarios in the next 20 years and literally no one is thinking about it, which does give me some doubt to these claims. Oh they are. The US Department of Defense has been incorporating Climate Change catastrophes into their operations planning for years, the ICEWARS program is designed to merge behavioral modeling and available data to identify points of conflict and unrest before they erupt so that they can be eliminated ahead of time, and there has been increasing militarization of police forces with a focus on crowd containment and disruption. If you are asking why they aren't trying to avert climate change, well, that goes into how big a shock to the system it would take to stop it. What half solutions we have can't be done fast enough to stop it, and investigating more will rile people up as more about the problem leaks out. Instead they are readying for pure triage when it all goes to poo poo, to hold onto control for as long as possible. People are most certainly planning for this. You just have to remember how godawful most people are and factor that in.
|
|
|
|
SGRaaize posted:This thread is making me depressed, but it really sounds unbelievable that people are suggesting catastrophic scenarios in the next 20 years and literally no one is thinking about it, which does give me some doubt to these claims. By the time we really fully understood what we were doing to the planet and how far we already screwed up it was to the point where we needed to slash 80% of our global industry in order to start dealing with it. That is just completely unpalatable to any government. When you add in the fact that so many politicians of every major country are in the pockets of various corporations which run those global industries it isn't just untenable, it's totally off the table. Instead they ignore it for now and we have some long term disaster plans to deal with the problem because we can't stop it.
|
|
|
|
Death Himself posted:By the time we really fully understood what we were doing to the planet and how far we already screwed up it was to the point where we needed to slash 80% of our global industry in order to start dealing with it. It's not even that sinister....human nature being what it is, there is no way whatsoever that you would ever get global cooperation. Some government, somewhere, would hold out for the technological advantage. It's no less unrealistic to believe that every country would dial back it's technological advantages during a time of peace than it is to believe that the same country would do so during a time of, say, global war. It's a global version of the monkey trap...and, at the end of the day, we're still monkeys. I cant even imagine what the actual "WAKE THE gently caress UP" event would be that would finally clear the air on this, but it wouldn't be soon enough to change. I hope I'm wrong and steeped in ignorance, but I fear that we've passed the tipping point.
|
|
|
|
PainterofCrap posted:I cant even imagine what the actual "WAKE THE gently caress UP" event would be that would finally clear the air on this, but it wouldn't be soon enough to change. I hope I'm wrong and steeped in ignorance, but I fear that we've passed the tipping point. We have, or will in the next 30 years. Hence the need to adopt nuclear, you phase out emissions (vehicles will be slowly replaced with electrics/mass transit, but grid energy needs a rapid switch). This will at least mitigate further damage. Advancing the extreme environment of space, instead of deep sea exploitation that's planned as the arctic circle opens; we could hope to slow our consumption of Earth's resources by establishing resource bases on the moon. Getting it started would be a monumental task, but it would go down in human history as one of our greatest moments
|
|
|
|
The biggest barrier to space/Moon colonization is the lack of gravity and the effect on the human body, specifically calcium loss. Humans simply cannot survive in a low-gravity environment for very long and still return to a gravity environment. If and when we ever develop an artificial gravity (or stick to centrifugal force to recreate 1G), we can revisit long-term space exploration. As it stands now, we can't even send a return manned mission to Mars.
|
|
|
|
PainterofCrap posted:The biggest barrier to space/Moon colonization is the lack of gravity and the effect on the human body, specifically calcium loss. Humans simply cannot survive in a low-gravity environment for very long and still return to a gravity environment. There's still no reason to suggest a person can't live in Lunar gravity for extended periods (they'd need to exercise though, as in microgravity) It's possible that on the moon regular exercise could prevent losses seen in zero G. Not that colonization is the main plan. We build plenty of cool robotics. I can see maybe a few hundred people living on the moon at any given time assembling modular tunneling/mining equipment launched from Earth, and then working alongside these machines (some of them remotely controlled from Earth). Older mines and existing caves can be sealed and pressurized, creating more comfortable habitats and places to experiment with turning regolith into soil.
|
|
|
|
Konstantin posted:There are positive feedback effects that keep this from happening. Once we cross a certain threshold, we are hosed, even if we reduce carbon emissions to zero afterwards. The Earth will probably recover on a time scale of thousands of years, but there is no recovery on a shorter time scale. The fun thing is we may be past the point of no return already, we just don't know it yet because of not knowing how much feedback effects affect our climate. There's a general C02 PPM level that we're not supposed to go past as a general rule and we're nearly there.
|
|
|
|
Pellisworth posted:I'm a chemical oceanographer working on my PhD. My thesis involves some stuff indirectly related to climate change, but I'm very familiar with ocean acidification, warming of the oceans, and other interactions between the atmosphere and oceans. I'm more than happy to (try and) answer any questions on those topics as well as give a little perspective as a scientist. Do you know much about methane clathrates? Those are what really freak me out. Ocean acidification is also bad, but I feel like there are ways we can mitigate it.. giant deposits of methane becoming soluble and boiling out of ice and the deep ocean would really gently caress us. The thing that makes me most angry about climate denial is the way people scoff and act smug in their denial. There are economic and health reasons that would make switching to efficient, clean technology a great idea. This combined with the scale of the potential disaster we are heading into tips the calculus from "waste of money if wrong" to "completely worthy pursuit even if we may be wrong in our predictions." ascii genitals fucked around with this message at 05:40 on Dec 8, 2011 |
|
|
|
PainterofCrap posted:The biggest barrier to space/Moon colonization is the lack of gravity and the effect on the human body, specifically calcium loss. Humans simply cannot survive in a low-gravity environment for very long and still return to a gravity environment. This is false, we only have data on 1g and 0g. A manned mission to mars would involve 500 day at martian g and a 0g travel time that has already been exceeded by astronauts on the ISS. The only obstacles to a manned returned mars mission are political. In any case centrifugal g is also a solved problem, you just have a tether between the ship and the spent upper stage booster and spin them.
|
|
|
|
Amoxicilina posted:And we have the audacity to think that climate change will make certain parts of the world more hospitable or manageable to us is ok. Once we all move up there, are we going to slash and burn the gigantic untouched boreal forests that exist in these places to grow corn and soybeans? Despite the fact that they provide a great quantity of the oxygen we need to breathe? It is even more complicated than that. Maybe eventually the arctic will be productive, but before that happened you'd have to get the soil acidity to usable levels. There are a lot of 6 foot tall spruce trees, and blueberries as tart as lemons growing in Alaska. http://en.wikipedia.org/wiki/File:World_Soil_pH.svg http://www.borealforest.org/index.php?category=world_boreal_forest&page=overview In the long run it would work, but, well, in the long run, you know. VideoTapir fucked around with this message at 06:11 on Dec 8, 2011 |
|
|
|
ascii genitals posted:
How does that help the people who are in power NOW, benefitting from the systems that would be displaced? They would bear much of the cost of such a transition, and to be sure, much of the benefit, but at the cost of creating opportunities for them to be displaced by new competitors.
|
|
|
|
VideoTapir posted:How does that help the people who are in power NOW, benefitting from the systems that would be displaced? They would bear much of the cost of such a transition, and to be sure, much of the benefit, but at the cost of creating opportunities for them to be displaced by new competitors. This is why we Occupy Oil, Coal and Gas Companies spend tons of money to maintain dominance of the energy market. They've played environmentalists like a fiddle against Nuclear. Fukushima is like God's gift to the oil execs to smear nuclear and make Deep Horizon go away.
|
|
|
|

|
| # ? Apr 30, 2024 19:27 |
|
PainterofCrap posted:It's not even that sinister....human nature being what it is, there is no way whatsoever that you would ever get global cooperation. Some government, somewhere, would hold out for the technological advantage. It's no less unrealistic to believe that every country would dial back it's technological advantages during a time of peace than it is to believe that the same country would do so during a time of, say, global war. It's a global version of the monkey trap...and, at the end of the day, we're still monkeys.
|
|
|