|
Dear microthread, please enjoy these pictures of my Ikea furniture and microscopy setup:  The photos show my most frequently-used setup, which is: - Olympus BH2 (BHT) microscope base - Nikon CFI BE 4x, 10x, 20x and 40x objectives (for observation), and an Olympus UIS2 UPlanFL N 10x / 0.3 for photography - Sigma quattro sd camera w/Takumar 150mm f4 lens - 3d-printed holders for retarders made of randomly-salvaged tape and cellophane wrapping It's a pretty cobbled-together combination, but it works well enough. The Olympus objective in particular is fantastic because it covers an APS-C sensor far better than its 26.5 field number would suggest, and the Nikon objectives are also good value for what they can do. Most of my photography is of the polarized-light kind (you can see my home-made 3d printed retarders in the first pic). I haven't done much in the last few months, I do have a couple of favourites from 2019 hanging up on my wall:   Hopefully I can get back into things this year. I've just finished my 14 days of self-isolation after returning to Sydney from three weeks in Japan, and I have a bunch of new (well, new to me) gear to try out! Dia de Pikachutos fucked around with this message at 14:01 on Apr 6, 2020 |
|
|
|

|
| # ¿ May 15, 2024 18:01 |
|
I do have the matching head, but one of those cases where it does optical corrections for the original objectives. I find it hard to use binocular heads anyway, so even if I'm just observing I tend to use camera for convenience. Tragically, Nikon's infinity system uses a larger objective format with 25mm threads and a 65mm parfocal length, rather than the 45mm DIN-standard RMS style that the BH2 takes. The CFI BE series is the only one that Nikon make that can be used on RMS scopes, and they only appear on the Eclipse E100 "education" microscope. The next model up, the E200, uses the same 25mm objectives as all of the bigger Nikon scopes. I'd love to get an old Nikon Eclipse, but scientific or medical surplus is virtually nonexistent in Australia since it seems like we stopped doing any sort of scientific research or manufacturing at the turn of the century in favour of over-finanicialising the economy and the commoditisation of residential real estate. Dia de Pikachutos fucked around with this message at 04:50 on Apr 7, 2020 |
|
|
|
Scarodactyl posted:I may try adapting one on if it will physically fit, though I guess I'd have to be pretty careful to avoid whacking it on the stage. Assuming that you mean adapting a 20x PlanAPO, it might be a bit tight, although I was wrong about the parfocal length of the PlanAPOs - they're 60mm parfocal length objectives, so the difference 15mm in comparison to the DIN 45mm ones. The BH2 stage has a fairly good range of movement (and the fine focus travel runs the full length of the mechanism). I guess you need at least 20mm of working room difference in objective parfocal length (60mm vs 45mm) plus a bit of extra whatever adapter you'd be using. I'll have a look when I get home to see what sort of wiggle room there is.
|
|
|
|
After checking just now it looks like you could just about adapt the CFI60 objectives onto the BH2. If I unlock the focus block and move it to its lowest possible position the clearance between the objective's shoulder and the stage is about 70mm, give or take.
|
|
|
|
Holy gently caress how much did you pay for that?Scarodactyl posted:I am still figuring it out. I'm getting some odd doubling on the images which I hope is fixable. The beamsplitter in the trinocular head might be the culprit, although take that with a grain of salt since I haven't ever used anything more complex than what I have now.
|
|
|
|
Sounds like you're spoiled for choice - I paid AU$1800 for my BH2/BHT with achromat objectives from a guy in an unsigned warehouse full of suspect-looking medical "surplus"!
|
|
|
|
Please please please give me a shout if you spot any Olympus UIS/UIS2 objectives going as surplus (particularly in the UPlanFL N series). You'd be like Microscope Jesus, assuming you'd be happy to take paypal $ and faff about with posting them down here. In other news, I've been testing the bucket of mounted achromats that I bought in Japan last month. The Sigma Life-Size Adapter is a pretty nice tube lens between 150 and 200mm from the camera sensor. Maybe even nicer than the 150mm and 200mm Takumars I've been using, since it seems to have less astigmatism: 
|
|
|
|
Yeah those are looking really good
|
|
|
|
Dear Microscope Thread, I hope you all like plant genitals. Shot with "stackula", which is an Arduino-based focus stacking controller project I have been working on in fits and spurts for a few months. I have an OG Stackshot, but the UI always gave me the shits because it has too many features and horrible buttons.   Stackula, on the other hand has:
Clicking the rotary encoder cycles between 3 modes: Coarse Focus  Fine focus  Stacking  Once you kick off a stack by pressing "up" on the rocker switch in Stacking mode, the rail moves from start to finish positions in the increments displayed for the currently-chosen numerical aperture. At each step the controller triggers the camera via a cannibalized shutter release cable & simple optocoupler circuit. If anyone wants the 3d files, source code for the controller and the [probably] complete BOM hit me up with a PM and I'll send them to you!
|
|
|
|
I probably need to add some sort of variable post-move wait time to allow for using continuous light sources - I use flashes myself so the only thing I need to consider is flash recycle time. At the moment the timings are fixed, but depending on requirement it's easy to change in the firmware since they're just constants in the config file. My current values are: 1. Move to next position 2. Wait 1500ms after moving to position (SETTLE_TIME_MS time) 3. Trigger shutter for 100ms (SHUTTER_TRIGGER_TIME_MS), and then wait for another 150ms (SHUTTER_REST_TIME_MS). 4. Wait another 1500ms after that (POST_MOVE_WAIT_TIME_MS) 5. Go to #1 e: I've PM'd you a link to the source so you can see what I mean Dia de Pikachutos fucked around with this message at 07:41 on May 15, 2021 |
|
|
|
I'm more of a sorcerer myself
|
|
|
|
Mmmhmm love me some DIC
|
|
|
|
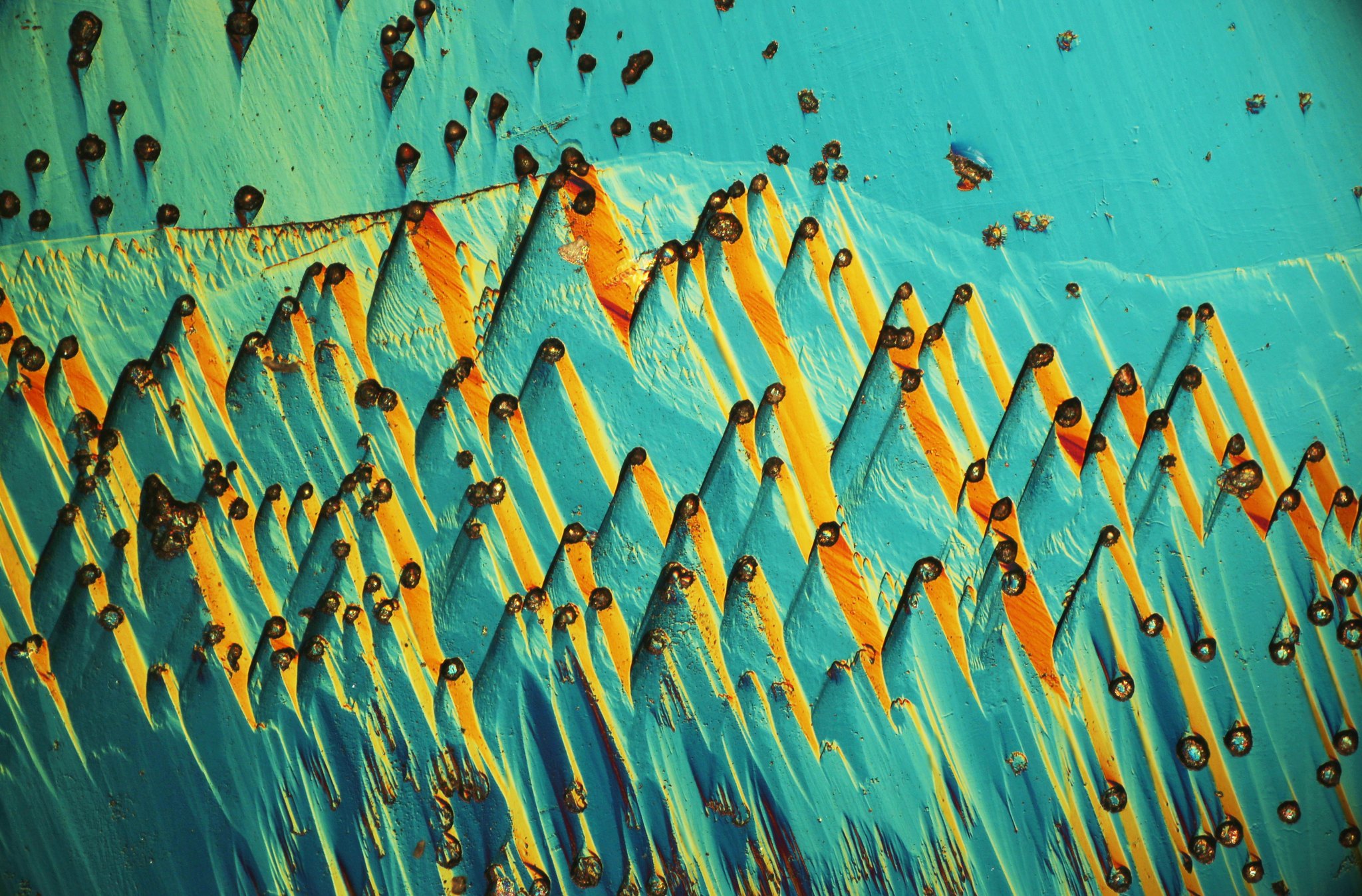
Scarodactyl posted:Belated, but thanks for the tips on that. I've been working on it but it's a lot to learn.  Amino acid crystals (Glutamine and Beta Alanine) Dia de Pikachutos fucked around with this message at 12:28 on Sep 14, 2021 |
|
|
|
It's okay, here is another picture so you don't feel bad. Same amino acids as above, just a lucky nucleation site I guess Dia de Pikachutos fucked around with this message at 12:10 on Sep 17, 2021 |
|
|
|

|
| # ¿ May 15, 2024 18:01 |
|
Reichert were actually one of the first companies to do infinity-corrected microscopes, from memory. They got absorbed by Leica Microsystems (different to the rich person accessory camera company), and also owned American Optical as well. Scarodactyl's post is extremely correct - BUT a stereomicroscope is probably not the best option for looking at single-celled critters. I may be wrong, though. I used to have a Leica ATC2000, which is a student microscope that's basically a slightly updated Reichert Series 160 / AO One Sixty. The 160's would be a very serviceable microscope for most pond critters, and might available on eBay for not that much more than the scope in your picture (if you are lucky). My ATC2000 was US$200, and another US$100 to ship to Australia. The US has a LOT of surplus microscope poo poo available at insane prices, so you might want to consider getting something shipped over. Most non-toy microscopes are modular, and have a set of components that can be switched in and out to support different microscopy techniques or price-points. For example, student scopes might have a monocular head, with a binocular head available as an option. It's generally best to assume that parts for one microscope model are not interchangeable with other models, even from the same brand. In any case, the things that you need in a transmitted-light microscope are (from bottom to top): - An electric lamp - typically part of the "stand" or "body" of the scope. - Coarse and Fine Focus knobs. This is extremely important. The "coarse" knob is basically what you use to move the lens turret out of the way when you change slides. It's pretty much useless for focusing. The "fine" knob is what you actually use when viewing a sample, and gives you very granular control. If a scope doesn't have coarse and fine knobs, then it's probably not what you want. - A "condenser" (which is basically a lens that collects light from the lamp and concentrates it onto a little circle on the slide that you can adjust the size of) - A "stage", which is what the slide goes on. Nicer stages have rack & pinion axes driven by knobs, which is really useful once you get to single-celled animal magnification. - Rotating objective turret with objectives. Typically 4x/10x/40x/100x on more recent scopes. You only really need 4x / 10x / 40x (see below). - A "head", which is where the eyepieces go. A binocular head is probably more comfortable for most people to use. - Eyepieces - usually 10x. 15x and 20x eyepieces are generally more expensive and uncomfortable to use, in my experience. As Scaro says, the total magnification is the objective magnification x the eyepiece magnification. For example, most scopes come with 10x eyepiece(s) - so with a typical set of 4x / 10x / 40x / 100x objectives, you get 40x - 1000x magnification. Older scopes might have a slightly different set of x numbers, like 5x / 10x / 43x - that's OK too. I'm pretty sure the 160's have infinity-corrected objectives - which are a more "modern" style of design. 40x magnification is enough magnification for: - Looking at the intimate details of most bugs - Other small things 4x objectives typically have the most "working distance" of the objectives on a scope (ie. the distance between the end of the objective and the slide). 4x objectives typically can have ~5mm (sometimes more) of working distance, so you might be able to use it to view "3d" objects that aren't mounted on a slide - like ants or grains of sand. You'll need to work out how to light them, though. 100x (10x objective w/ 10x eyepiece) is enough magnification to see: - Really big single-celled critters (some stentor are ~1mm long) - Very small worms like aeolosoma (related to earthworms) and flatworms (their own thing) - Other very small animals like rotifers, insect larvae, the embryos in snail eggs 10x objectives will typically have about 1-2mm of working distance. 400x is enough of magnification to see: - blood cells, - single-celled critters like amoebas, euglena, lacrymaria, paramecium & stentor, - single-celled plants like desmids, diatoms and algae - really tiny multicellular animals like tardigrades (water bears) and rotifers 40x objectives will typically have about 0.5mm of working distance. Dia de Pikachutos fucked around with this message at 09:50 on May 21, 2023 |
|
|