|
DemeaninDemon posted:Radium also really fucks up your forums. In 1600 years or so we'll be calling him radon.
|
|
|
|

|
| # ? May 31, 2024 22:57 |
|
WarpedNaba posted:You forgot beta radiation, chum. That's another form of ionizing radiation you don't want near you. It has little penetrating power, though. Beta emitters inside you are far more dangerous than ones lying around on the ground. I used to have a watch that used tritium as a back light. The beta particles would make the phosphor glow continuously, but they couldn't get out of the watch case itself.
|
|
|
|
WarpedNaba posted:You forgot beta radiation, chum. That's another form of ionizing radiation you don't want near you. Yeah, but that's handily just a case of "don't ingest or go near anything emitting beta radiation". It's not like gamma which'll loving SNIPE you if you let it.
|
|
|
|
Deteriorata posted:It has little penetrating power, though. Beta emitters inside you are far more dangerous than ones lying around on the ground. I used to have a watch that used tritium as a back light. The beta particles would make the phosphor glow continuously, but they couldn't get out of the watch case itself. Are you thinking of alpha? Alpha radiation is the stuff that gets stopped by your dead skin layer but fucks up your insides. Beta is the stuff that can travel a meter or so, and generally the benchmark for absorbation is aluminium plates. I don't think it would be stopped by a few millimetres of glass, for a start.
|
|
|
|
It depends entirely on how energetic they are, but normally a beta particle can be stopped by a sheet of aluminum foil. Your skin or a piece of paper will stop an alpha particle. Gamma rays require large quantities of lead to block. Neutrinos require several lightyears of lead to be effectively blocked, according to something I read a couple years ago... but fortunately they basically don't interact with anything else either, and really don't matter healthwise.
|
|
|
|
WarpedNaba posted:Are you thinking of alpha? Alpha radiation is the stuff that gets stopped by your dead skin layer but fucks up your insides. Beta is the stuff that can travel a meter or so, and generally the benchmark for absorbation is aluminium plates. I don't think it would be stopped by a few millimetres of glass, for a start. Tritium is a very low-energy beta emitter; the electrons have about the same kinetic energy as typical alpha particles. Beta radiation from tritium only penetrates about 5 mm of air.
|
|
|
|
It's one of the few commercially available radioactive materials that are still completely unrestricted. I got a nice little tritium light on my key chain. It's a tiny tube (1 mm thick, 4-5 mm long) filled with tritium, covered in a fluorescent material, which sits in the middle of a ~10 mm thick plastic cylinder. The online store I got it from said these lights are intended as fishing bait, but you can use them for whatever. They're safe, and their half-life is a bit over 10 years so they'll last long enough. Looks like a tiny point of light in the dark, like a firefly. A light without an off switch is a bit unusual, though.
|
|
|
|
kastein posted:It depends entirely on how energetic they are, but normally a beta particle can be stopped by a sheet of aluminum foil. Your skin or a piece of paper will stop an alpha particle. IIRC, the rule of thumb is that one light-year of solid lead will block about 50% of neutrinos. They're so weakly interacting that they're impossible to shield against, but there's no need to -- they won't interact with you any more than they would with your shielding.
|
|
|
|
wouldn't a light-year-long volume of solid lead just collapse into a singularity pretty quick 
|
|
|
|
I'm sure you'd get more harm from working with a lightyear thick block of lead than you would from the 50% of neutrinos it stopped.
|
|
|
|
Tollymain posted:wouldn't a light-year-long volume of solid lead just collapse into a singularity pretty quick Depends how thick it is.
|
|
|
|
James Garfield posted:Tritium is a very low-energy beta emitter; the electrons have about the same kinetic energy as typical alpha particles. Beta radiation from tritium only penetrates about 5 mm of air. It penetrates so poorly that the only viable way to measure tritium in water is to mix it with an organic scintillant (a fluorescent compound that emits visible light after being excited by the beta particles) and then measuring the flashes of light with a photomultiplier. Liquid scintillation counters are actually fascinatingly accurate in spite of the rather roundabout way of detection. I did a lab last year where we took samples from municipal water supplies and found a clear relationship between tritium concentration and proximity to our nearby CANDU power plants. However, it was still on the order of 30-80 Bq/L when the regulatory limit is 1000 Bq/L. As an aside if you ever drink tritiated water the standard way of figuring out your dose is to collect a urine sample and run it through an LSC. Teehee.
|
|
|
|
BattleMaster posted:It penetrates so poorly that the only viable way to measure tritium in water is to mix it with an organic scintillant (a fluorescent compound that emits visible light after being excited by the beta particles) and then measuring the flashes of light with a photomultiplier. Liquid scintillation counters are actually fascinatingly accurate in spite of the rather roundabout way of detection. I did a lab last year where we took samples from municipal water supplies and found a clear relationship between tritium concentration and proximity to our nearby CANDU power plants. However, it was still on the order of 30-80 Bq/L when the regulatory limit is 1000 Bq/L. Tritiated water is the same as heavy water, right? Does that have any health effects, or do enzymes that require water just not notice the difference and keep on doing their thing?
|
|
|
|
Icon Of Sin posted:Tritiated water is the same as heavy water, right? Does that have any health effects, or do enzymes that require water just not notice the difference and keep on doing their thing? Heavy water uses the second isotope of hydrogen, deuterium (D). Each deuterium nucleus has a mass of 2, so the molar mass of heavy water is 20, rather than 18 for normal water. And yes, it does make a difference biologically. There's something called the Kinetic Isotope Effect which is essentially that heavier isotopes of an element are moving more slowly and thus diffuse and react more slowly than the lighter isotopes. For most elements, the difference is not enough to matter. Deuterium is twice the mass of protium, though, so it moves roughly 40% slower. This is enough to disrupt many biological reactions, and if you drank nothing but heavy water you would die.
|
|
|
|
But how would it taste. Fakeedit: VVVVV Oh, well alright then.
|
|
|
|
I think you need to replace half your body with heavy water (or something like that) before it becomes problematic, though. It's more notable that it tastes a little sweet.
|
|
|
|
Icon Of Sin posted:Tritiated water is the same as heavy water, right? Does that have any health effects, or do enzymes that require water just not notice the difference and keep on doing their thing? No, they're not the same. Tritiated water is water where one or both of the hydrogen atoms is tritium instead of protium or deuterium. Heavy water is water where both of the hydrogen atoms are deuterium instead of protium (and semiheavy water is where only one is deuterium). The major distinction from a health point of view is that tritiated water is radioactive and so is dangerous if ingested in relatively small quantities. Heavy water is dangerous if drank in large quantities over time but this isn't really a danger outside of laboratory experiments or thought exercises because of the expense and rarity of heavy water. Heavy water and semiheavy water occur naturally in a small quantity and a few different processes exist for separating it from light water but it's expensive to perform. Tritiated water is primarily produced in nuclear power plants and especially heavy water reactors like CANDUs when a deuterium atom in water absorbs a neutron. Ontario Power Generation operates a tritium removal facility for processing used heavy water from their CANDU plants where the tritium is stored in sealed capsules, but some still escapes the plants and is monitored by the CNSC. To my knowledge there has never been a major tritiated or heavy water spill in Ontario. Sometimes demineralized water is spilled and this causes an uproar in the media because of fear mongering targeting the nuclear industry but it poses no environmental problems. Losing demineralized water is mostly just lost money for the plant because of how expensive it is to operate the on-site treatment plant. Edit: Demineralized water used in the secondary coolant loop (where the steam is made, run through turbines, and then condensed) has some hydrazine added to it to keep the dissolved oxygen down. Hydrazine is toxic and sometimes steam is released into the atmosphere on purpose as a safety mechanism but the levels of hydrazine are low and also carefully monitored. BattleMaster has a new favorite as of 06:53 on Jan 10, 2015 |
|
|
|
This made me think about something. The decay of tritium is H-3 --> He-3 + e- + neutrino The e- is the beta radiation, of course, and flies away from the reaction site. Only a few mm through water, but on a molecular scale that's easily far enough to say that particular electron is gone from the reaction. Beta electrons are formed in the nucleus and aren't related to the radioactive isotopes' orbital electrons. What we're left with is a positively charged He+ ion (it still has the orbital electron from the hydrogen, but didn't gain any and needs two now). This is one of the most unstable ions I can think of and will steal an electron from anything. For instance, Wikipedia says that TH (a hydrogen gas molecule with a single tritium atom) forms HeH+ when it decays. Helium doesn't want to be in a compound, so the H+ will bond to anything, making this an incredibly strong acid. I would expect something similar to happen in tritiated water. Now, Wikipedia says that when hit by radiation, water will decompose into hydroxide radicals (dangerous), hydrogen peroxide (dangerous) and oxygen compounds such as ozone (dangerous). Alpha and beta particles cause this, which is why pure tritium oxide is corrosive. It doesn't say what happens to decayed the decayed THO molecule, though. I'm thinking of the case where it's diluted in the water in a human body. My guess is that the 'He+' will quickly take an electron from either the remaining OH or from its surroundings. In the latter case it'll form a He atom (harmless), a radical from the molecule it reacted with (dangerous), and an OH. radical (dangerous). In the former case (more likely, as the OH is there anyway), it also forms a harmless He atom, and what's left over must be an OH+ ion. This OH+ ion is also going to be very unstable and short-lived. It would probably react as an incredibly strong Lewis acid with whatever it finds (dangerous). For instance, with water, it would be OH+ + H2O --> H2O2 + H+. The H+ just makes the water a bit more acidic but the cell can deal with that. The hydrogen peroxide is, again, dangerous. If the OH+ reacts with some organic molecule, the results could be worse. Now I'm wondering if in the human body, this whole set of energetic reactions of the decay product doesn't cause more harm than whatever that beta-particle did. It's something that's never really brought up when talking about radioactivity. Carbon dioxide has a new favorite as of 11:51 on Jan 10, 2015 |
|
|
|
Carbon dioxide posted:Now I'm wondering if in the human body, this whole set of energetic reactions of the decay product doesn't cause more harm than whatever that beta-particle did. It's something that's never really brought up when talking about radioactivity. You may be right but it's much easier to quantify the health effects of ionizing radiation because the decay energy of tritium is known and the activity can be determined by tests. It isn't my field, but I can't imagine that it's easy to figure out how spontaneously-forming strong acids hurt someone other than "it's bad."
|
|
|
|
You know what else has penetrating power?
|
|
|
|
 Ruthenium compounds?
|
|
|
|
Carbon dioxide posted:
No, that's really how ionizing radiation causes cellular damage. The odds of an alpha particle or a beta particle actually striking a DNA molecule in a cell are pretty darn low. But if one smacks into a water molecule in one of your cells, it can kick an electron free and ionize it. So now you have an electron floating loose and an H2O+ , which can then react with another H2O to give you H3O+ and an HO- radical. The electron can in turn react with oxygen to form an oxygen radical. These free radicals can go on to react with your DNA. It's definitely not just the ionizing radiation itself that causes cell damage, it's the chemical reactions that follow when the radiation ionizes parts of you. Phanatic has a new favorite as of 04:21 on Jan 11, 2015 |
|
|
|
SeaGoatSupreme posted:
Nonsense, that's a penis.
|
|
|
|
DemeaninDemon posted:You know what else has penetrating power? Dickite? 
|
|
|
|
SeaGoatSupreme posted:
What's the K here?
|
|
|
|
Say Nothing posted:Dickite? 
|
|
|
|
please don't post my Tinder picture
|
|
|
|

|
|
|
|
Phanatic posted:No, that's really how ionizing radiation causes cellular damage. The odds of an alpha particle or a beta particle actually striking a DNA molecule in a cell are pretty darn low. But if one smacks into a water molecule in one of your cells, it can kick an electron free and ionize it. So now you have an electron floating loose and an H2O+ , which can then react with another H2O to give you H3O+ and an HO- radical. The electron can in turn react with oxygen to form an oxygen radical. These free radicals can go on to react with your DNA. Yeah and the number of ionization events caused by the beta particle can be predicted if you know the energy of it*. Since multiple ionizations are likely to occur per beta particle then the damage caused by tritiated water forming acids after decay is likely to be much smaller. *the energy of radioactive decay products is measured in the first place with a proportional counter by using the same logic in reverse, which I think is really neat
|
|
|
|
Oh, I didn't think of one beta particle causing multiple ionizations.
|
|
|
|
Over in the scoodenfroody thread, this got posted:NoneMoreNegative posted:Re: plaster - this gave me the shudders when I read it originally, no schadenfreude here though I once got a cast of my nose made for a sculpture (silly high school student art project). This involved lying on a table while a friend glopped plaster of Paris all over my face and letting it cure, and while it definitely did get warm, I still have a nose. What went wrong, chemically speaking, here? I (kinda) get the whole exothermic thing. I remember schmearing my face in Vaseline so I wouldn't have to endure an unintentional wax job while prying the mold off; would that make a difference? Is it the direct contact, or the encasing, or...? Fake edit: Here's a BBC article with no gross pictures and kinda goes into "larger the mass, bigger reaction". But I still don't get how you'd burn eight of your fingers off with stuff they let us mess around with in high school art class. http://news.bbc.co.uk/2/hi/uk_news/education/6485481.stm Not fake double edit: jeebus, to be an art student and lose your hands. And not the only one, according to that BBC article. Plus one goon in that thread mentioned knowing of a gal who lost a finger. I never knew plaster of Paris was so drat dangerous! My art teacher gave me a bunch at the end of the school year and I molded my own hands in my parents basement, just for kicks, that summer.  Kinda terrifying to think I could've potentially lost all my digits back then. Kinda terrifying to think I could've potentially lost all my digits back then.
JacquelineDempsey has a new favorite as of 18:24 on Jan 22, 2015 |
|
|
|
"In addition, it is used in relatively thin layers so the heat can dissipate. In bulk, the temperature can reach 60°C or so."
|
|
|
|
Wasabi the J posted:"In addition, it is used in relatively thin layers so the heat can dissipate. In bulk, the temperature can reach 60°C or so." Yeah, I read that. Guess I'm just still thrown by what "relatively thin" vs "bulk" is, since the cast of my nose was easily 1 or 2 inches at its thickest point, and all I remember was it getting "oooh, it's a bit hot under this", not "oh god my face is searing off". Same when I did my hands; as I recall (I am
|
|
|
|
People make wax casts of their hands all the time, like I did in grade school, but somehow there are people who come out of it with 2nd degree burns. Weird, huh?
|
|
|
|
I have a story I like to share. It's not about dangerous chemicals, per se. But it is about chemistry, and I think it is funny. When the Euro was introduced back in 2002, the European Central Bank made sure to put a whole lot of new security features in the notes. I believe there are around three dozen in any note. Some of them, everyone knows. The 'sprinkles' you can only see under UV light. The watermark. And so on. But many security features are only known by a limited amount of people, some are only known by the ECB and perhaps the national central banks themselves. One of these used to be the unique spectroscopic signature. For those who don't know: spectroscopy is an analysis method where you find out what substances are in a sample by either measuring light absorption or light emission. It's even better if you include non-visible EM waves such as infrared and ultraviolet. It's the main way astronomers find out about the composition of far-away stars and planets, and it's a very powerful analysis technique in chemistry. Now, the prof who taught the first years' spectroscopy class told us that he wanted to know if Euro notes have interesting spectroscopic features, so he did some tests himself. Turns out, they do. They contain a small amount of a lanthanide. Lanthanides are lanthanum and the long row of elements that come after it in the periodic table. If you see two rows sitting below the table apart from the rest, the top row contains the lanthanides. Lanthanides each have their own unique spectroscopic signature, that can't be faked by using other compounds. They are also known as 'Rare Earths', and while they aren't terribly rare, they aren't that common either. So, using a lanthanide creates a security feature that's hard to fake and easy to detect. Great! The question is... which lanthanide? If each has their own signature, it doesn't really matter, right? It doesn't. But I'm sure some people at the European Central Bank had a good chuckle when they came up with this one. The lanthanide used for the spectroscopic signature of Euro notes is... Europium, of course! Carbon dioxide has a new favorite as of 09:25 on Jan 23, 2015 |
|
|
|
Carbon dioxide posted:It doesn't. But I'm sure some people at the European Central Bank had a good chuckle when they came up with this one. The lanthanide used for the spectroscopic signature of Euro notes is... Europium, of course. America can only answer this by doping bills with Americium.
|
|
|
|
America will just rename Europium to Libertium
|
|
|
|
JacquelineDempsey posted:Over in the scoodenfroody thread, this got posted: Something something fingerless mittens
|
|
|
|
Conversation in the pub about Californium Astatine (CfAt2, using 211At, 252Cf) as a possible "what's the most batshit compound we can come up with" (no, it's probablynot possible, but fun to think about). I needed a refresher in just what astatine entails. Per wiki: quote:All of its isotopes are short-lived; the most stable is astatine-210, with a half-life of 8.1 hours. Okay, that's pretty cool.
|
|
|
|

|
| # ? May 31, 2024 22:57 |
|
I particularly like the Wikipedia article for Einsteinium.quote:The high radioactivity of einsteinium-253 produces a visible glow and rapidly damages its crystalline metal lattice, with released heat of about 1000 watts per gram... Einsteinium is the element with the highest atomic number which has been observed in macroscopic quantities in its pure form, and this was the common short-lived isotope einsteinium-253. 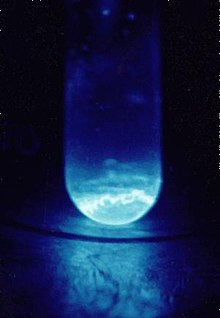
|
|
|
























 for healed up hands-minus-fingers.
for healed up hands-minus-fingers.